eefit依飛把「半服務、半零售」的經營模式引入商場服務市民的健康品牌,希望提供一種在「家樓下」的服務。查詢分店網絡













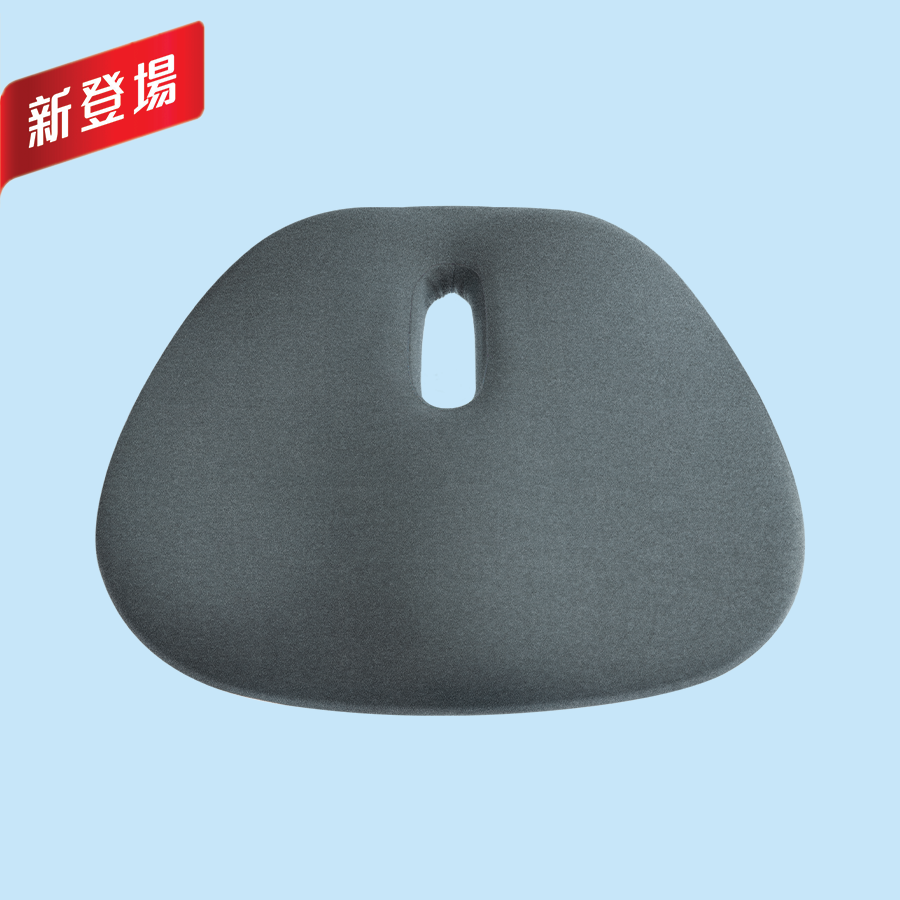
eefit Energy Cushion
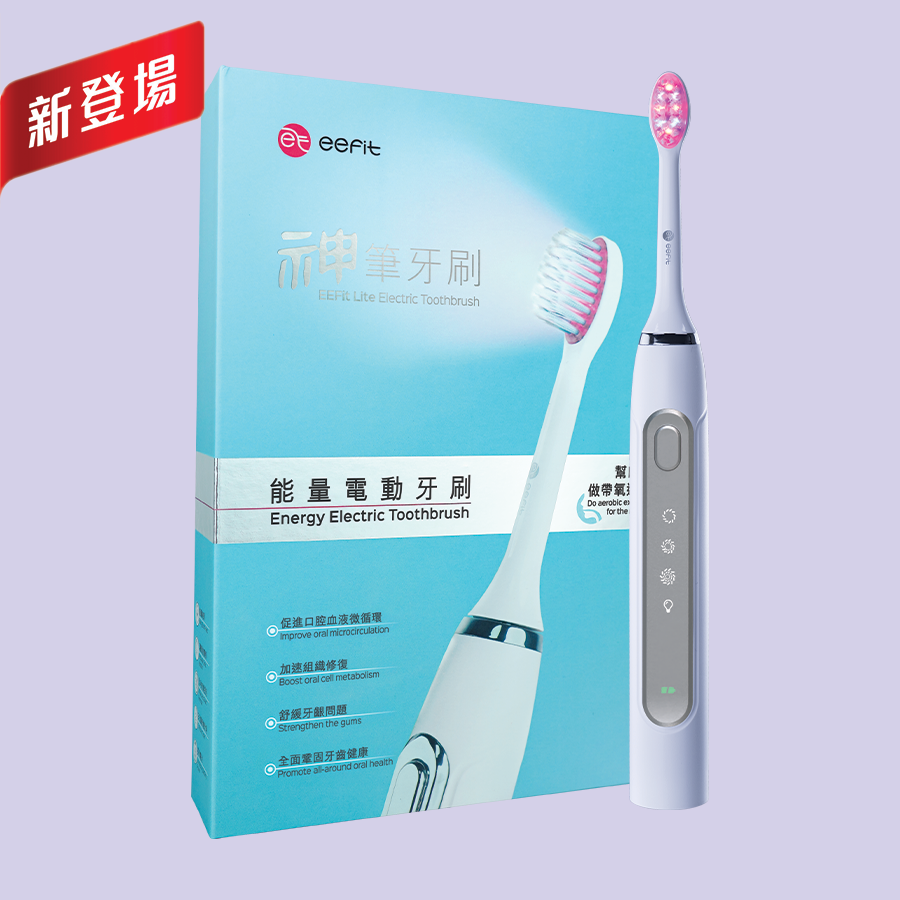
EEFit Lite Electric Toothbrush
Aqua Vitaliser
Energy-Cleansing Gel_Homepage-Hero_Desktop_ZH@2x-1-scaled
Energy-Lipstick_Homepage-Hero_Desktop_ZH@2x-1-scaled
Energy-Nail-Care_Homepage-Hero_Desktop_ZH@2x-1-scaled
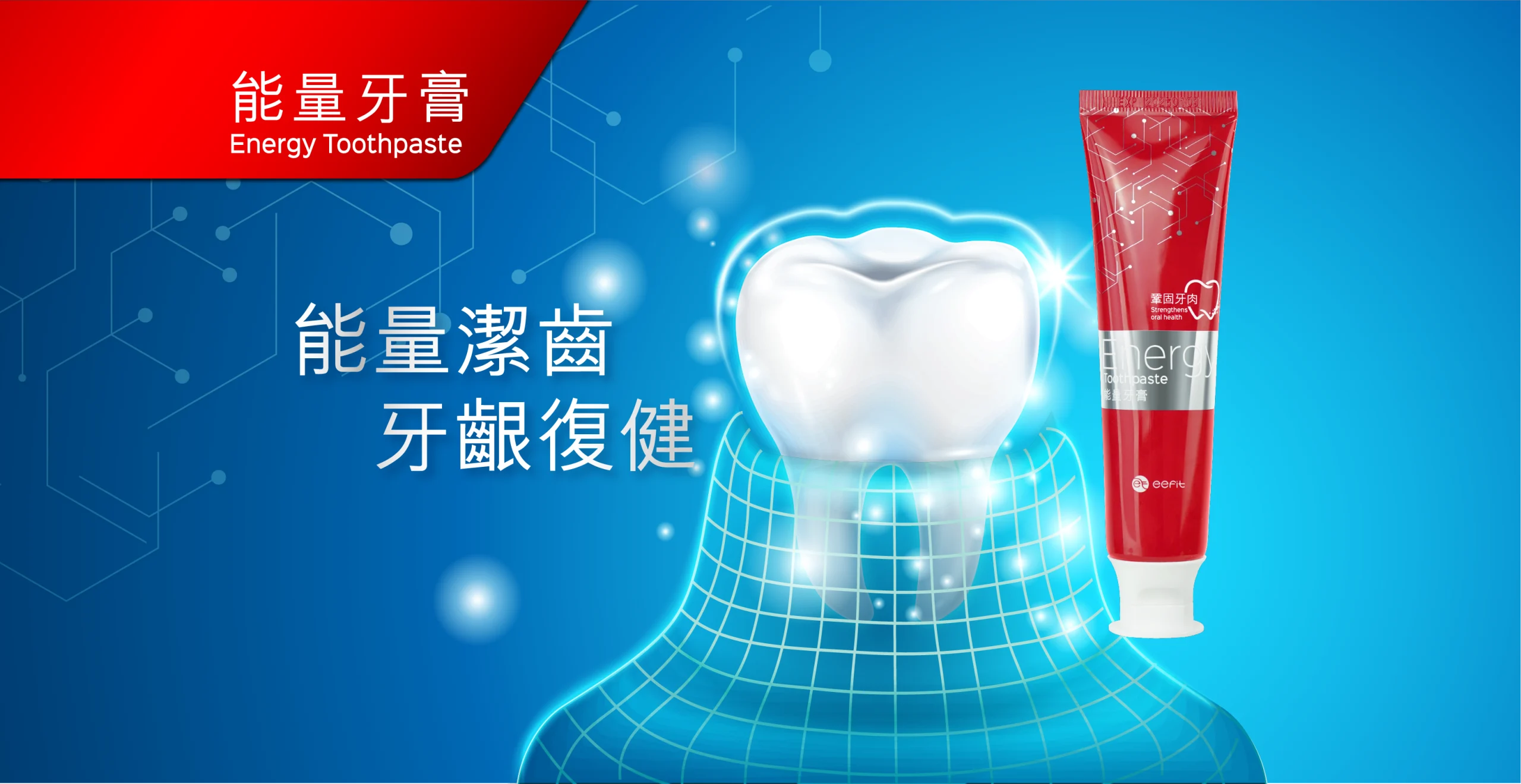
Hero-Banner_PC@2x-scaled
Hero-banner_PC_1@2x-1-scaled
Energy-Mattress-Pad-Related_Homepage-Hero_Desktop_ZH-6@2x-scaled
Hero-banner_PC_ZH@2x-scaled
Energy-Velvety-Pillow-Sheet_Home-Hero_Desktop@2x-100-1-copy-scaled
Ultra-Health-Light-Related_Homepage-Hero_Desktop@2x-100-1-scaled-copy
Ultra-Health-Light-Related_Homepage-Hero_Desktop@2x-100-1-scaled-copy
AquaenergyD-1-copy













eefit Energy Cushion
EEFit Lite Electric Toothbrush
Aqua vitaliser
Energy-Cleansing Gel_Homepage-Hero_Mobile_ZH@2x
Energy-Lipstick_Homepage-Hero_Mobile_ZH@2x
Energy-Nail-Care_Homepage-Hero_Mobile_ZH@2x
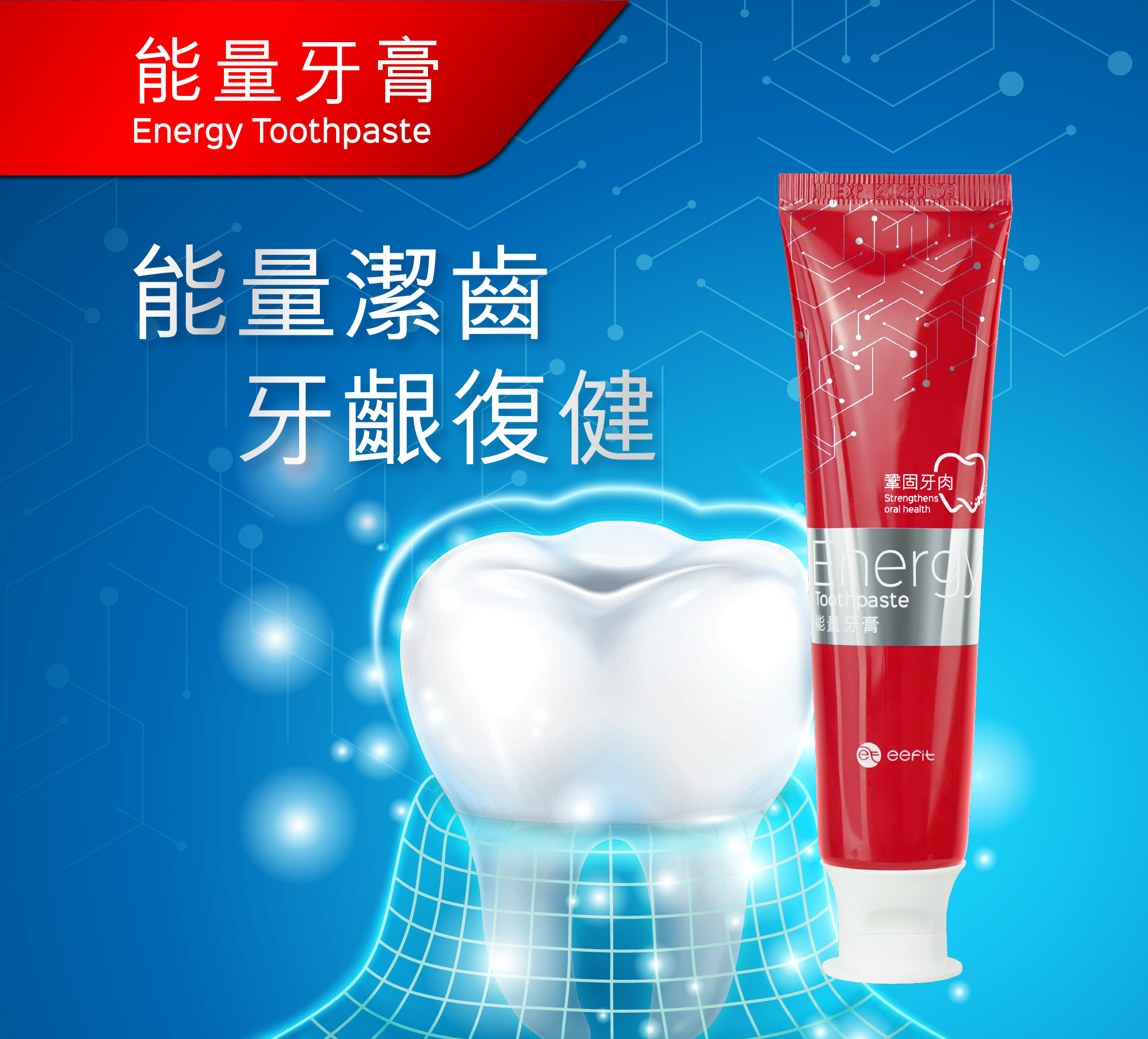
Hero-Banner_mobile@2x
Hero-banner_mobile_1@2x-1
Energy-Mattress-Pad-Related_Homepage-Hero_Mobile_ZH-@2x
Hero-banner_mobile_ZH@2x
Hero-Banner_mobile@2x
Ultra-Health-Light-Related_Homepage-Hero_Mobile@2x-100-1-copy
Ultra-Health-Light-Related_Homepage-Hero_Mobile@2x-100-1-copy
Hero-Banner_mobile@2x